Written by SmartSense
Key takeaways
Most non-refrigerated pharmaceuticals come with the recommendation on the package that you store them in a “cool, dry place.” Continuous and automated temperature monitoring makes it easy to follow the “cool” part of these recommendations by changing the temperature of a cooler or freezer when alerted. Wisconsin’s Board of Pharmacy has recently become the first to make a recommendation on how to interpret the “dry” part of the recommendation.
Their regulation reads:
“Wis. Adm. Code Phar 6.075: Temperature; Humidity
1 (b) “Dry place” means a place that does not exceed 40 percent average relative humidity at 68 degree Fahrenheit or the equivalent water vapor pressure at other temperatures.”
At first glance, this is a little complicated. While most are familiar with the discomfort that accompanies a hot and humid summer day, for example, many people don’t know what the water vapor pressure is at any temperature—or how it can vary with temperature.
Water vapor pressure is the contribution of water molecules to the air pressure. It increases with the water content of the air as well as with temperature. Relative humidity is the comparison of the actual water vapor pressure to the maximum water vapor pressure that the air can have at a given temperature before it condenses. This is why relative humidity cannot be above 100 percent (at which point is starts to rain).
As the temperature of air goes up, so does the maximum water vapor pressure, but this relation is not linear. The figure below shows the relationship between water vapor pressure, temperature, and relative humidity. Colors correspond to water vapor pressure in mmHG. The black lines are isobars, a.k.a. lines of constant pressure. The thickest line corresponds to 7 mmHG, or the water vapor pressure equivalent to 40 percent humidity at 68℉—the maximum allowed by the new Wisconsin regulations. The other three isobars (at 3, 5, and 9 mmHG) show how non-linear the relationship is between the three factors.
Figure 1 - The relationship between water vapor pressure, temperature, and relative humidity. Blue corresponds to low water vapor pressure and black is high water vapor pressure. The black lines are isobars—lines of constant pressure—with the thick line equalling 7 mmHG, the maximum water vapor pressure allowed by the new WI regulation.
From this figure, we can see that below certain temperatures, the water vapor pressure will never exceed the definition of “dry” in the statute. The temperature/humidity combination at which the thick black line hits 100 percent humidity is not at risk of degrading pharmaceuticals, despite the air holding the maximum amount of water possible at that temperature. While this allows dryness to be ignored in coolers (“cold and dry” places), “cool and dry” generally refers to pharmaceuticals stored at room temperature in warehouses and retail locations. We can probably all agree that 40℉ is not a comfortable temperature for a room!
We have found temperatures in these environments to commonly range from 60–80℉, which allows for a maximum relative humidity that can be anywhere from 50 percent in a cooler room to 26 percent in a warmer room. This huge variation means we need to track not only temperature and relative humidity, but also water vapor pressure.
Figure 2 shows two stores in WI over eight days in November. The red line is the temperature, the blue line is the relative humidity, and the purple line is the water vapor pressure. The vertical purple lines are readings where the water vapor pressure exceeds the regulations. Both show spikes in humidity and water vapor pressure on the afternoon of November 15, corresponding to a snow and rain shower that hit around that time.
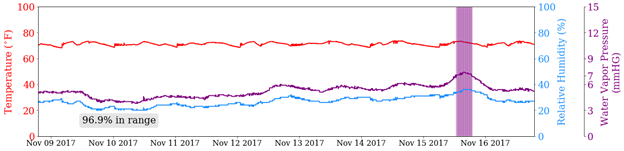
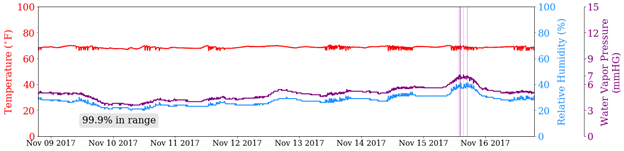 Figure 2 - The temperature (red), relative humidity (blue), and water vapor pressure (purple) timelines for two stores in WI during an eight-day span in November 2017. The vertical purple lines represent water vapor pressure readings that exceeded the mandated threshold.
Figure 2 - The temperature (red), relative humidity (blue), and water vapor pressure (purple) timelines for two stores in WI during an eight-day span in November 2017. The vertical purple lines represent water vapor pressure readings that exceeded the mandated threshold.
On this particular week, no store we monitored in Wisconsin was in compliance less than 96.9 percent of the time. While this is a very good statistic, November in Wisconsin is not known for its high levels of humidity. This could be a very different picture in the summer. One could also argue that the phrase “average relative humidity… or equivalent water vapor pressure” indicates that the average water vapor pressure must remain below the threshold, which all of the stores did.
Similar to temperature, not every excursion will matter by itself. We should instead consider a cumulative effect, where pharmaceuticals kept in stores that are consistently out of range—or that experience large excursions—are in more danger of going bad.
Subscribe to Our Blog!
Interested in learning more? Subscribe to our blog to get the latest posts about remote monitoring and pharmaceutical compliance.
Topics:
Subscribe to the SmartSense Blog
Stay up-to-date on the evolution of IoT connectivity.
