Written by SmartSense | Pharmacy
Key takeaways
Please visit the CDC website for information on when you will be eligible to receive the COVID-19 vaccine.
As the third wave of the COVID-19 pandemic sweeps over the country from coast to coast, the recent news from Pfizer and Moderna of a successful vaccine candidate is the good news we’ve all been waiting to hear. When the pharmaceutical companies get final FDA approval, they will face two enormous challenges: one, making millions of doses available for timely administration; and two, keeping those doses cold and potent during transportation, storage, and handling.
 Like all vaccines, new COVID-19 vaccines will be temperature and time sensitive. They will require massive adjustments to cold chain and storage cooler capacity. Why? Because the current inventory of all existing vaccines (especially flu) and temperature-sensitive pharmaceuticals already in the supply chain will need to make room for the huge influx of COVID-19 vaccine doses.
Like all vaccines, new COVID-19 vaccines will be temperature and time sensitive. They will require massive adjustments to cold chain and storage cooler capacity. Why? Because the current inventory of all existing vaccines (especially flu) and temperature-sensitive pharmaceuticals already in the supply chain will need to make room for the huge influx of COVID-19 vaccine doses.
Fortunately, the temperature monitoring technology that SmartSense by Digi has perfected over the past ten years will be on the front lines to protect this precious inventory. Some of our clients have been chosen by Operation Warp Speed as pharmacies taking the lead in local distribution and administration during Phase 1.
Our technology is already in place at these pharmacies to meet CDC-mandated continuous temperature monitoring of the COVID-19 vaccine inventory. Not only that, our comprehensive IoT solutions are built to support every link along the entire cold supply chain, including research labs, factories, warehouses, trucks, and a variety of other distribution outlets.
OPERATION WARP SPEED: A Phased Approach
Operation Warp Speed (OWS) is the acting partnership among the Department of Health and Human Services, the Department of Defense, the Centers for Disease Control and Prevention (CDC), and companies in the private sector to develop, manufacture, and distribute COVID-19 vaccines, therapeutics, and diagnostics.
The principal objective of OWS is to produce and administer enough vaccines for all U.S. citizens who choose to be vaccinated. OWS will make maximum use of all healthcare professionals licensed to administer vaccines, including allied health professionals such as pharmacists. OWS is implementing its strategy to distribute and administer the COVID-19 vaccine in three phases.
The following graph illustrates the phases of the COVID-19 Vaccine Program.
SOURCE: https://www.cdc.gov/vaccines/imz-managers/downloads/COVID-19-Vaccination-Program-Interim_Playbook.pdf
Phase 1: Potentially Limited Doses Available
Upon FDA approval, the first batch of vaccine doses will be distributed using a focused strategy. OWS will ensure its selected vaccination locations reach targeted critical populations while managing cold chain requirements. Pharmacies will be on the front lines of administration along with other closed point-of-distribution (POD) settings that allow for the maximum number of people to be vaccinated while maintaining social distancing protocols.
Phase 2: Large Number of Vaccine Doses Available
When greater quantities of vaccine become available, OWS has two simultaneous objectives:
- Provide widespread access to vaccination and achieve coverage across the entire population
- Ensure uptake in target populations, particularly those who are at high risk for severe outcomes from COVID-19
Phase 3: Continued Vaccination, Shift to Routine Strategy
If the risk of COVID-19 persists, vaccines will be universally available and integrated into routine vaccination programs, run by both public and private partners.
PUBLIC SECTOR DISTRIBUTION: McKesson Corporation
At the direction of OWS, McKesson Corporation will expand its existing partnership with CDC’s Vaccines for Children Program (VFC) to serve as the federal government’s centralized distributor of COVID-19 vaccines. CDC and McKesson previously collaborated in response to the H1N1 pandemic in 2009-2010.
McKesson is the largest seasonal flu vaccine distributor in the U.S. and distributes up to 150 million doses of all vaccines annually to public health clinics, hospitals, physician offices, nursing homes, pharmacies, and other care facilities.
Centralized distribution allows the government full visibility, control, and ability to shift assets and use data to optimize vaccine uptake. Once vaccines are allocated to an authorized partner, McKesson will deliver a specific amount of vaccines to a designated location.
SOURCE: https://www.hhs.gov/sites/default/files/strategy-for-distributing-covid-19-vaccine.pdf
PRIVATE SECTOR DISTRIBUTION: Pfizer Inc.
Pfizer has announced it is preparing its vaccine candidate for distribution in high hopes that it earns FDA approval. If so, the pharma firm plans to bypass McKesson and other distribution wholesalers and distribute the vaccinations directly. Company officials say they will deliver up to 100 million doses this year and another 1.3 billion in 2021.
In Kalamazoo, Michigan, Pfizer has built a huge staging facility the size of a football field outfitted with 350 large freezers. Here, their vaccine inventory will be stored and eventually transported on dozens of cargo-jets and hundreds of trucks every day. Total delivery time, from distribution center to point of use, is expected to be an average of three days.
To keep the vaccines safe during transit, Pfizer designed a reusable container that can maintain ultra-cold temperatures for up to 10 days. These suitcase-sized boxes will be packed with dry ice and tracked by GPS.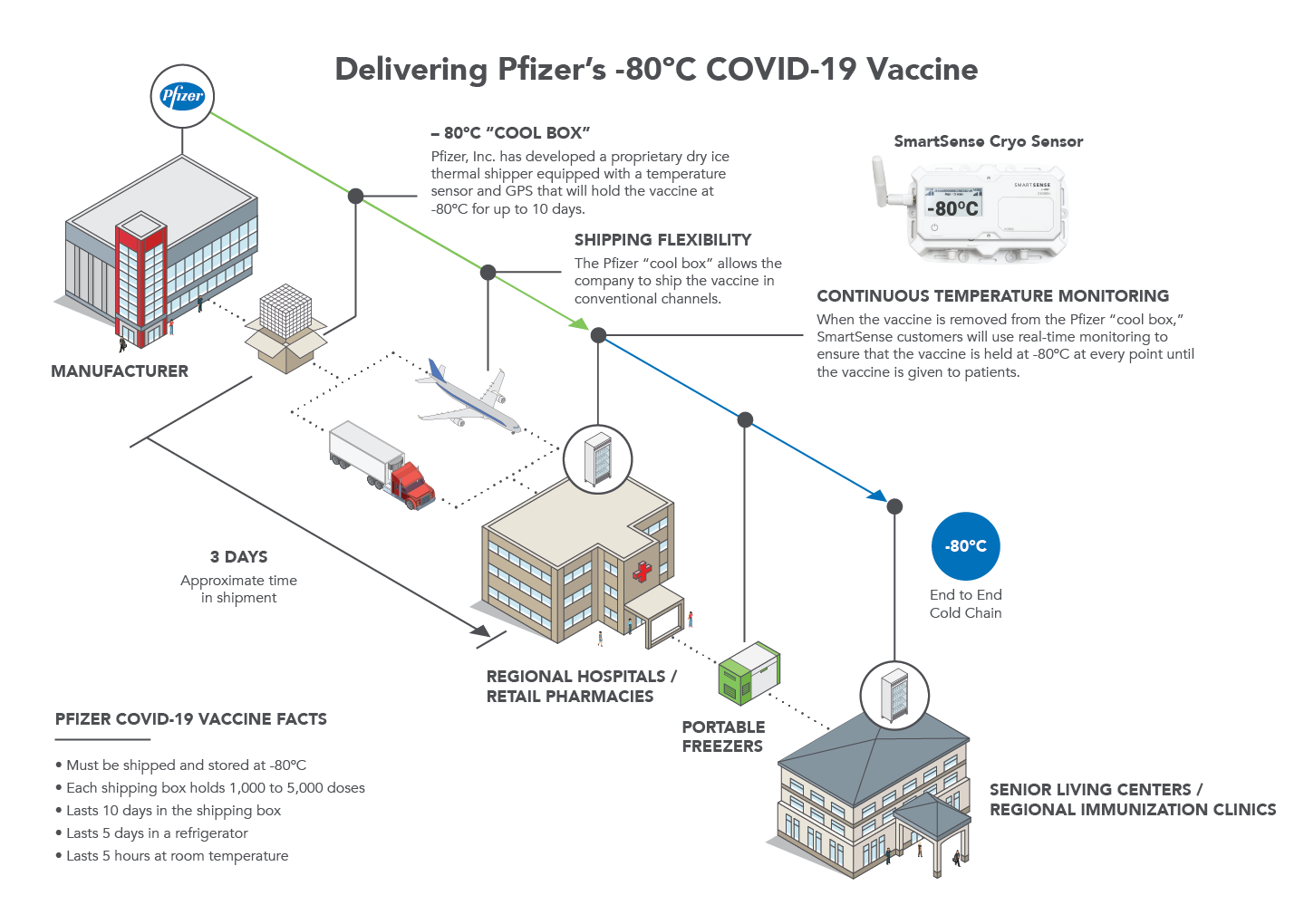
Source: www.smartsense.co
TRANSPORTATION: Air & Ground Carriers
After distribution locations and schedules are determined, the next challenge is for the national transportation network to deliver the vaccines to administration sites. At this time, Delta, UPS, and FedEx are preparing for COVID-19 vaccine shipments, both air and ground.
Delta has expanded its cooler facilities at its Atlanta warehouse and will use a special container in cargo holds of its planes to ship vaccines. This Opticooler is now part of the airline’s cold chain pharmaceutical program to transport vaccines. It uses a battery that operates up to 120 hours and can maintain temperatures from 2-8 degrees Celsius or 15-25 degrees Celsius without dry ice.
UPS is building two giant freezer farms capable of super-cooling millions of vials of a COVID-19 vaccine, preparing for when the company will need to transport millions of vials at high speed. The facilities, under construction in Louisville, Kentucky, will house 600 deep freezers that can each hold 48,000 vials of vaccines at temperatures as low as -80 Celsius (-112 Fahrenheit).
CDC STORAGE & HANDLING REQUIREMENTS
COVID-19 products must be stored and handled correctly to ensure potency and maximize shelf life. Overall, it is expected that cold chain storage and handling requirements for all potential COVID-19 vaccine products will vary in temperature from refrigerated (2°C to 8°C) to frozen (-15 to -25°C) to ultra-cold (-60°C to -80°C).
For this reason, the CDC has required that continuous temperature monitoring must be conducted along every link in each vaccine’s cold supply chain. The chain begins at the manufacturing plant, includes delivery to and storage at the vaccination provider site, and ends with administration to the general population. Vaccination providers, including pharmacies, will be responsible for maintaining vaccine quality from the time a shipment arrives at a vaccination provider site until the dose is administered.
SMARTSENSE BY DIGI: Monitoring Vaccines for Phase I Pharmacies
OWS has contracted with three national pharmacy chains who will be first-wave POD administration sites which also happen to be current clients. SmartSense technologies, in fact, collect more than 10 million sensor readings per day in 28,000 retail pharmacy locations and their supporting distribution centers.
“Our pharmacy customers already have entrusted us to protect more than $2 billion in refrigerated inventory,” says Kevin C. Riley, President of SmartSense by Digi. “In addition, we’re also working with hospitals, clinics, and transportation companies that use our technology for similar applications.”
The CDC is expected to mandate continuous temperature monitoring for these pharmacies to receive and distribute forthcoming COVID-19 vaccines. SmartSense by Digi monitoring solutions meet the CDC guidelines. Our automated wireless-monitoring and task-management tools not only ensure vaccines remain at the proper temperature, but also that workers handle the inventory properly.
“We’ve spent years developing and continuously improving the SmartSense solution to help our customers protect their cold chain for critical, condition-sensitive inventory,” says Riley. “This has prepared our customers for the monumental challenge of storing and distributing a COVID-19 vaccine and has helped Digi build relationships with some of the largest organizations that are set to play key roles in getting the vaccine to the public.”
SmartSense is a proven, trusted solution. We’re proud to provide greater certainty to both our customers and the public that, when a COVID-19 vaccine is ready, it will safely and reliably reach them with full efficacy.
Topics: Pharmacy
Other Suggested Posts
How to Ensure the Integrity of Life Sciences Supply Chains
According to an SAP survey from 2022, a majority of senior executives at enterprise businesses reported that the unprecedented supply chain disruptions caused ...
How High Reliability Organizations Evaluate IoT Vendor Security Compliance
Focusing more attention recently on patient-centered care, the U.S. healthcare sector has been motivated to establish more high reliability organizations ...
How to Improve Asset Health of Medical Refrigeration
Table of Contents: What is asset health management? How to ensure the safety and potency of medical products Pros of digitalizing and automating medical ...
Subscribe to the SmartSense Blog
Stay up-to-date on the evolution of IoT connectivity.



