Written by SmartSense
Key takeaways
At the end of June 2016, the U.S. Center for Disease Control (CDC) released an updated Vaccine Storage and Handling Toolkit. Among the most interesting changes is the new recommended Fahrenheit temperature range for refrigerated vaccines. The Fahrenheit temperature range for refrigerated vaccines was updated from 35°F - 46°F to 36°F - 46°F. The Celsius temperature range remains unchanged: 2°C - 8°C.
The CDC notes, ”The conversion of 2°-8° Celsius equates to 35.6°- 46.4° Fahrenheit. Among routinely recommended vaccines, most manufacturers’ package inserts note a Fahrenheit temperature range of 36°F - 46°F, while some note a temperature range of 35°F - 46°F. The Fahrenheit temperature range of 36°F - 46°F aligns with guidance from the United States Pharmacopeia (USP), a scientific organization that sets federally recognized quality standards for food and drugs.”
The change reflects the fact that Celsius and Fahrenheit temperature conversions do not generally result in whole numbers. 2°C becomes 35.6°F and 8°C becomes 45.4°F. The 2014 Toolkit with the lower recommendation of 35°F makes the threat of freezing the vaccine and compromising the safety and effectiveness more likely to occur.
What's the big deal? It's only a difference of 0.6°F.
It may seem small but that 0.6°F brings vaccines that much closer to 32°F, the temperature at which water freezes and vaccines could be damaged. Often vaccines contain live organisms that could be killed if frozen. Keeping a safer distance from 32°F is a good idea. This is particularly true for pharmacies, medical offices, and hospitals that rely on twice per day manual temperature monitoring and paper logs. These sites would be more likely to miss events where refrigerator cooling cycles cause the temperature to dip and as a result make the danger of freezing more likely.
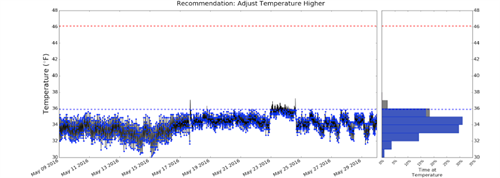
Continuously monitored refrigerator temperature readings show how cooling cycles can bring internal temperatures below freezing (32°F).
In the figure above, the refrigerator is running below 36°F most of the time. If the twice daily manual temperature readings both occurred at the top of the defrost cycle, this unit would be considered in compliance even though in actuality it is running below the suggested temperature range most of the time. To further complicate the issue, opening and closing the refrigerator door throughout the day can increase the air temperature within the fridge so everything would appear to be fine but in reality overnight temperatures could be dropping below freezing.
Raising the recommendation from 35°F to 36°F will not solve the problem completely but it will provide a greater safety cushion for proper vaccine storage, and in doing so keep vaccine recipients safer and better protected.
Topics:
Subscribe to the SmartSense Blog
Stay up-to-date on the evolution of IoT connectivity.