Written by SmartSense
Key takeaways
Many U.S. state regulations for pharmacies recommend monitoring prescription refrigerators and freezers twice per day, manually. This entails, a pharmacist reading a thermometer inside a fridge or freezer and recording it on a paper log, at the beginning and the end of the day. These daily logs are kept in files at the pharmacy for review during inspections. In the CDC Vaccine Storage and Handling Toolkit, the “CDC recommends the use of a continuous monitoring and recording digital data logger (DDL) with a current and valid Certificate of Calibration Testing (also known as a Report of Calibration), set at a minimum recording interval of at least every 30 minutes.”
Left: Traditional paper temperature logs (VFC Toolkit image).
Right: Wireless temperature sensor reads temperatures every 15 minutes, transmits data to gateway link to Cloud based data logs.
While twice per day logs are acceptable, they may not represent the full picture. Think of the adage, "a broken clock is correct twice per day." If you are only looking at single points in time, you could be missing something.
Below are temperature graphs from a Rx refrigerator in an operating pharmacy. Temperature readings are taken every 15 minutes and wirelessly transmitted to a cloud-based remote monitoring platform. The first graph displays the temperatures for a one-hour period. The temperature appears very stable between 41°F and 42°F. The CDC Toolkit recommends a range of 36°F - 46°F (2°C - 8°C) for Rx refrigerators. The unit is operating well within the recommended range. Everything is fine.
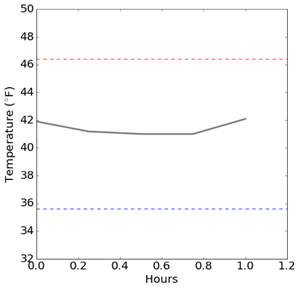
Above: Pharmacy Rx refrigerator temperatures readings for a one hour period.
The second graph (immediately below) shows one day of temperature readings in the same Rx refrigerator. While the fridge is within the CDC limits most of the day, an elevated temperature event is very noticeable at around 18 hours. Which means the medications were subjected to temperatures above the recommended 46°F for a short period. If temperatures were being recorded manually, this excursion would most likely have been missed. In this case, the pharmacy is aware of the event and took the appropriate corrective action.
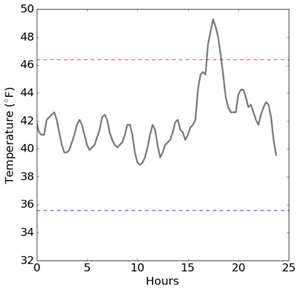
Above: Same pharmacy Rx refrigerator temperatures readings for a one day period.
The third graph (immediately below) shows one week of temperature readings in the same Rx refrigerator. Multiple events are visible between about 140 hours to 160 hours. Manual record keeping may have caught one of these excursions, but it's unlikely it would have caught all of them. With continual monitoring, they have a record of the temperature profile of this unit and are better able to link excursions to pharmacy activity. These cycles may be due to regular changes in pharmacy operations. For example, perhaps there is a lot of activity toward the end of the week where the refrigerator door is opened multiple times to fill prescriptions. If this is the case, pharmacists may be able to take additional steps to reduce elevated readings.
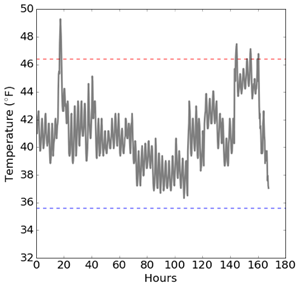
Above: Same pharmacy Rx refrigerator temperatures readings for a one week period.
Temperature cycles in refrigerators are expected to occur. When the refrigerator warms, the compressor turns on to cool it down. When doors are open, temperatures rise. These events would be lost using manual temperature monitoring and could potentially jeopardize the safety and efficacy of medications. Don't be in the dark with manual temperature monitoring. Get the insights you need and take control of your pharmacy operations.
Topics:
Subscribe to the SmartSense Blog
Stay up-to-date on the evolution of IoT connectivity.