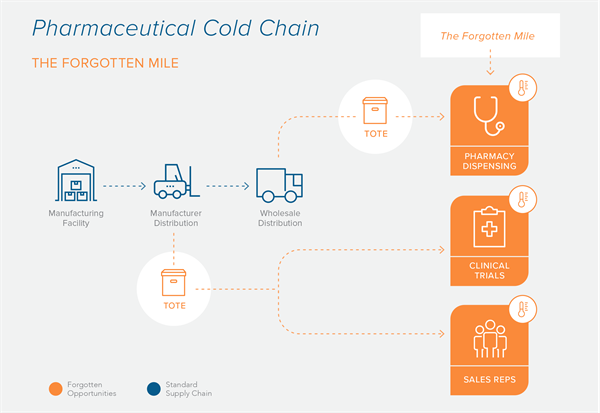
The CDC recommends that medications be kept within specific refrigerated and frozen temperature ranges to ensure safety and efficacy (Vaccine Storage and Handling Toolkit).
Drug manufacturers are well aware of these recommendations and take precautions to properly store their medications. However, there is a link in the Cold Chain that is often overlooked. We refer to that missing link as the “Forgotten Mile.”